| 目录: 12268 |
| 产品名称: Human C2 Protein |
| 规格: 10 µg, 50 µg and 100 µg |
| 基因符号: CO2;ARMD14 |
| Target: C2 |
| UNIPROT ID: P06681 |
| 描述: Recombinant human C2 Protein with C-terminal 6xHis tag |
| 背景: Component C2 is a serum glycoprotein that functions as part of the classical pathway of the complement system. Activated C1 cleaves C2 into C2a and C2b. The serine proteinase C2a then combines with complement factor 4b to create the C3 or C5 convertase. Deficiency of C2 has been reported to associated with certain autoimmune diseases and SNPs in this gene have been associated with altered susceptibility to age-related macular degeneration. This gene localizes within the class III region of the MHC on the short arm of chromosome 6. Alternative splicing results in multiple transcript variants encoding distinct isoforms. Additional transcript variants have been described in publications but their full-length sequence has not been determined.[provided by RefSeq, Mar 2009] |
| Species/Host: HEK293 |
| Molecular Weight: The protein has a predicted molecular mass of 81.9 kDa after removal of the signal peptide. The apparent molecular mass of C2-His is approximately 100-130 kDa due to glycosylation. |
| Molecular Characterization: C2(Ala21-Leu752) 6×His tag |
| 纯化:: The purity of the protein is greater than 85% as determined by SDS-PAGE and Coomassie blue staining. |
| Formulation & Reconstitution: Lyophilized from nanodisc solubilization buffer (20 mM Tris-HCl, 150 mM NaCl, pH 8.0). Normally 5% – 8% trehalose is added as protectants before lyophilization. |
| 储存和运输: Store at -20°C to -80°C for 12 months in lyophilized form. After reconstitution, if not intended for use within a month, aliquot and store at -80°C (Avoid repeated freezing and thawing). Lyophilized proteins are shipped at ambient temperature. |
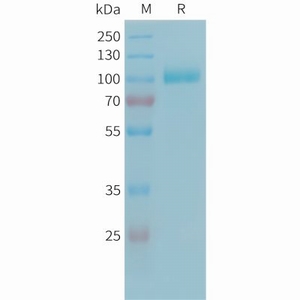
Figure 1. Human C2 Protein, His Tag on SDS-PAGE under reducing condition. |











