| 目录: 11571 |
| 产品名称: Human AGR2 Protein |
| 规格: 10 µg, 50 µg and 100 µg |
| 基因符号: AG-2;hAG-2;HPC8 |
| Target: AGR2 |
| UNIPROT ID: O95994 |
| 描述: Recombinant human AGR2 protein with C-terminal human Fc tag |
| 背景: This gene encodes a member of the disulfide isomerase (PDI) family of endoplasmic reticulum (ER) proteins that catalyze protein folding and thiol-disulfide interchange reactions. The encoded protein has an N-terminal ER-signal sequence, a catalytically active thioredoxin domain, and a C-terminal ER-retention sequence. This protein plays a role in cell migration, cellular transformation and metastasis and is as a p53 inhibitor. As an ER-localized molecular chaperone, it plays a role in the folding, trafficking, and assembly of cysteine-rich transmembrane receptors and the cysteine-rich intestinal gylcoprotein mucin. This gene has been implicated in inflammatory bowel disease and cancer progression. [provided by RefSeq, Mar 2017] |
| Species/Host: HEK293 |
| Molecular Weight: The protein has a predicted molecular mass of 44.0 kDa after removal of the signal peptide. The apparent molecular mass of AGR2-hFc is approximately 35-55 kDa due to glycosylation. |
| Molecular Characterization: AGR2(Arg21-Leu175) hFc(Glu99-Ala330) |
| 纯化:: The purity of the protein is greater than 95% as determined by SDS-PAGE and Coomassie blue staining. |
| Formulation & Reconstitution: Lyophilized from nanodisc solubilization buffer (20 mM Tris-HCl, 150 mM NaCl, pH 8.0). Normally 5% – 8% trehalose is added as protectants before lyophilization. |
| 储存和运输: Store at -20°C to -80°C for 12 months in lyophilized form. After reconstitution, if not intended for use within a month, aliquot and store at -80°C (Avoid repeated freezing and thawing). Lyophilized proteins are shipped at ambient temperature. |
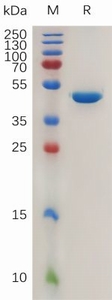
Figure 1. Human AGR2 Protein, hFc Tag on SDS-PAGE under reducing condition. |











